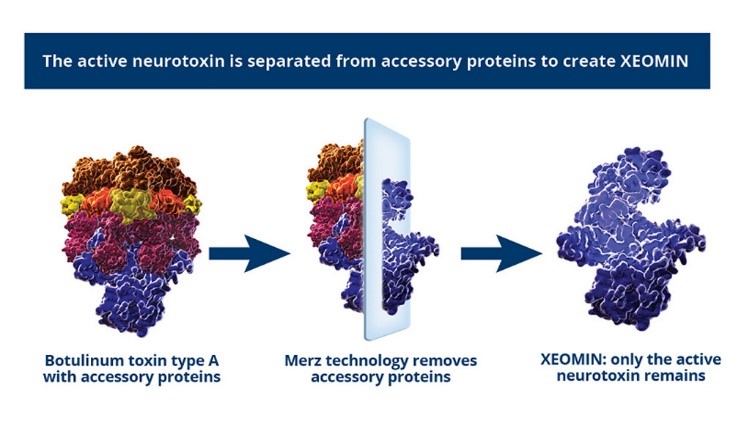
The Only Pure Neurotoxin Free From Complexing Proteins
XTRACT TechnologyTM is the only state-of-the-art manufacturing process that uniquely purifies the molecule removing the unnecessary proteins, leaving just the active therapeutic component.
XEOMIN® Patients Did Not Develop Clinical Resistance Due to Neutralizing Antibodies (NAb)
· Of the 1490 patients treated with XEOMIN® in placebo-controlled clinical trials across all indications, no patients demonstrated clinical resistance or secondary treatment failure due to Nab.
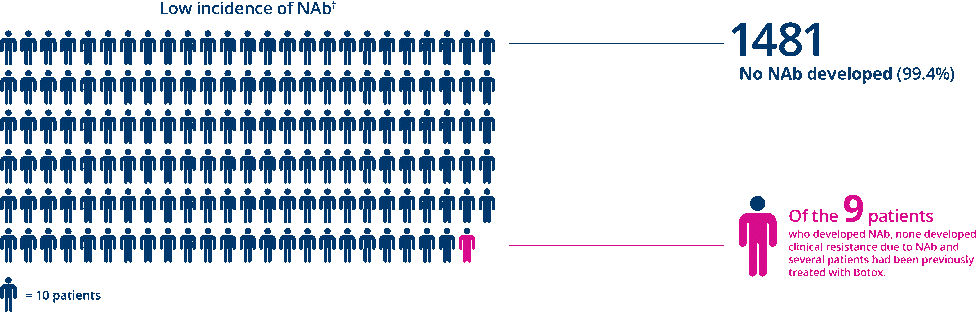
XEOMIN® Has a Molecular Weight of 150 kDa
- The proprietary manufacturing process of XEOMIN® isolates the active neuromodulator from the accessory proteins and reduces the number of proteins.
- XEOMIN® is formulated to have high biological activity with a low protein load.
Indications and Usage:
XEOMIN® is FDA approved for the treatment of adult patients with:
- Glabellar Lines
- Blepharospasm
- Cervical Dystonia
- Upper Limb Spasticity
- Chronic Sialorrhea
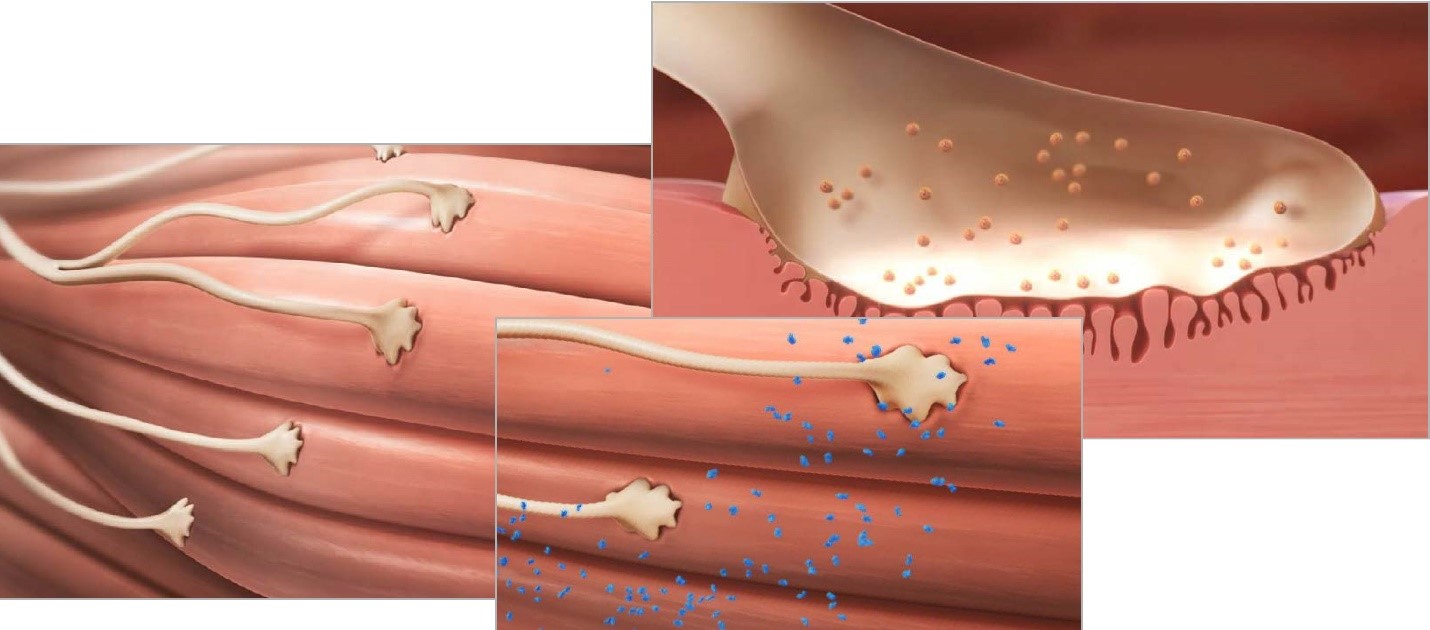
XEOMIN® Mechanism of Action
XEOMIN® is a botulinum toxin type A that blocks transmission at the neuromuscular junction by inhibiting the release of acetylcholine from peripheral cholinergic nerve endings.
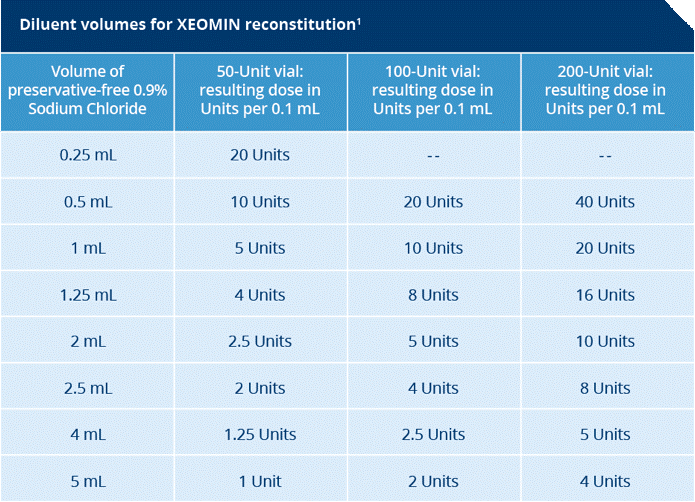
Reconstitution and Dilution: Flip It. Don't Shake It.
- Prior to injection, reconstitute each vial of XEOMIN® with sterile, preservative-free 0.9% Sodium Chloride Injection.
- A 20- to 27-gauge short-bevel needle is recommended for reconstitution
- Draw up an appropriate amount of preservative-free 0.9% Sodium Chloride Injection, USP into a syringe
Step 1: Vial preparation
Clean the exposed portion of the rubber stopper of the vial with alcohol (70%) prior to insertion of the needle.
Step 2: Saline injection
After vertical insertion of the needle through the rubber stopper, the
vacuum will draw the saline into the vial. Gently inject any remaining saline into the vial to avoid foam formation. If the vacuum does not pull the saline into the vial, then XEOMIN® must be discarded.
Step 3: Mixing
Remove the syringe from the vial and mix XEOMIN® with the saline by carefully swirling and inverting/flipping the vial —do not shake vigorously.
Reconstituted XEOMIN® is a clear, colorless solution free of particulate matter. XEOMIN® should not be used if the reconstituted solution has a cloudy appearance or contains floccular or particulate matter.
Storage: No Refrigeration Required
- Refrigeration of unopened vials is not required
- Unopened vials of XEOMIN® should be stored at or below 25°C (77°F)
- Do not use after the expiration date on the vial
- Reconstituted XEOMIN® may be stored in a refrigerator at 2° to 8°C (36° to 46°F) for up to 24 hours until time of use tains floccular or particulate matter.
Shelf life
An unopened XEOMIN® vial can be stored for up to 4 years at room temperature (up to 25°C)
Administration Instructions
- Intended for intramuscular or intra-salivary gland injection only.
- Should be used for only 1 injection session and only 1 patient.
- For intramuscular injections, the number of injection sites is dependent upon size of muscle to be treated and volume of reconstituted XEOMIN® injected. A suitable sterile needle (eg, 27–30 gauge [0.30–0.40 mm diameter], 12.5 mm length) should be used for intra-salivary gland administration for the treatment of chronic sialorrhea.
- The salivary glands can be located using ultrasound imaging or surface anatomical landmarks.
- Reconstituted XEOMIN® solution should be administered within 24 hours after dilution. During this time period, reconstituted XEOMIN® should be stored in a refrigerator at 2° to 8°C (36° to 46°F).